We are interested in novel synthesis strategies, novel compounds and nanomaterials, their analytical characterization and material properties. Aiming at material properties, we address structure, morphology, shape, luminescence, imaging, drug delivery, catalysis, photocatalysis, conductivity, magnetism, sorption.
Figure: Overview of our research activities.
Some of our major achievements are:
- 2024: [GeRu6(CO)18HI]: A Germanium-Centered Ruthenium Carbonyl Cluster with Aromatic Ring Current. S. Wolf, R. Köppe, J. Treptow, W. Feuerstein, J. Wenzel, F. Breher, P. W. Roesky, F. Weigend, W. Klopper, C. Feldmann*. Adv. Sci. 2024, accepted.
- 2023: High-Load Gemcitabine Inorganic-Organic Hybrid Nanoparticles as Image-
Guided Tumor-Selective Drug-Delivery System To Treat Pancreatic Cancer. M. Ischyropoulou, K. Sabljo, L Schneider, C. M. Niemeyer, J. Napp*, C. Feldmann*, F. Alves*, Adv. Mater. 2023, 2305151(1-15) - 2023: Amorphous Drug Nanoparticles for Inhalation Therapy of Multidrug-Resistant Tuberculosis. D. Rudolph, N. Redinger, K. Schwarz, F. Li, G. Hädrich, M. Cohrs, L. A. Dailey,* U. E. Schaible*, C. Feldmann*, ACS Nano 2023, 17, 9478-9486.
- 2021: 18-Crown-6 Coordinated Metal Halides with Bright Luminescence and
Nonlinear Optical Effects. E. Merzlyakova, S. Wolf, S. Lebedkin, L. Bayarjargal, B. L. Neumeier, D. Bartenbach, C. Holzer, W. Klopper, B. Winkler, M. Kappes, C. Feldmann*, J. Am. Chem. Soc. 2021, 143, 798–804. - 2021: Liquid-Phase Synthesis of Highly Reactive Rare-Earth Metal Nanoparticles. D. Bartenbach, O. Wenzel, R. Popescu, L.-P. Faden, A. Reiß, M. Kaiser, A. Zimina,
J.-D. Grunwaldt, D. Gerthsen, C. Feldmann*, Angew. Chem. Int. Ed. 2021, 60, 17373-17377. - 2018: Au@Nb@HxK1-xNbO3 Nanopeapods with Near-infrared Active Plasmonic
Hot-Electron Injection for Water Splitting. Y.-C. Chen, Y.-K. Hsu, R. Popescu, D. Gerthsen, Y.-G. Lin, C. Feldmann*, Nature Commun. 2018, 9, 232:1–11 - 2013: Ammonia-in-Oil-Microemulsions and Their Application. F. Gyger, P. Bockstaller, D. Gerthsen, C. Feldmann*, Angew. Chem. 2013, 125, 12671–12675; Angew. Chem. Int. Ed. 2013, 52, 12443–12447
- 2011: [C4MPyr]2[Br20] − Ionic Liquid based Synthesis of the first three-dimensional Polybromide Network. M. Wolff, J. Meyer, C. Feldmann*, Angew. Chem. 2011, 123, 5073–5077; Angew. Chem. Int. Ed. 2011, 50, 4970–4973
- 2009: Microemulsion Approach to Nanocontainers and its Variability in Composition and Load. H. Gröger, F. Gyger, P. Leidinger, C. Zurmühl, C. Feldmann*, Adv. Mater. 2009, 21, 1586–1590
- 2003: Polyol-mediated Preparation of nanoscale Oxide Particles. C. Feldmann, H. O. Jungk, Angew. Chem. 2001, 113, 372-374; Angew. Chem. Int. Ed. 2001, 40, 359–362
For the synthesis of novel compounds and high-quality nanomaterials, we have different liquid-phase methods available to address specific conditions and requirements:
- Polyol synthesis (synthesis in high-boiling alcohols)
- Ionic-liquid-based synthesis
- Liquid-ammonia-based synthesis
- Microemulsion techniques
- Solvothermal methods
- Aqueous synthesis
Hollow Nanopheres
Hollow nanospheres are intensely discussed regarding their properties (e.g., large specific surface, low specific weight, container-type morphology) and their functions (e.g., catalysis, gas storage, low-weight building materials, drug delivery). We have established a novel microemulsion-based strategy to obtain hollow nanospheres. In contrast to standard microemulsion techniques, the reaction was not performed inside a micelle, but at its phase boundary. The as-prepared hollow nanospheres exhibit outer diameters of 20 to 50 nm, a wall thickness of 3 to 20 nm and an inner diameter of 10 to 30 nm (Figure 1). These hollow nanospheres are specifically interesting for drug delivery (e.g., INH@Fe2O3 hollow nanospheres, INH: isoniacide) for antibiotic tuberculosis therapy (Figure 2).
Beside the microemulsion approach, we have established a synthesis strategy based on NaCl templates that can be easily removed after hollow-sphere synthesis by washing with water (Figure 3). Our activities, especially, focus on the following aspects:
- Synthesis / characterization of hollow nanospheres
- Variation of size, surface area, porosity
- Catalysis
- Drug delivery and drug release
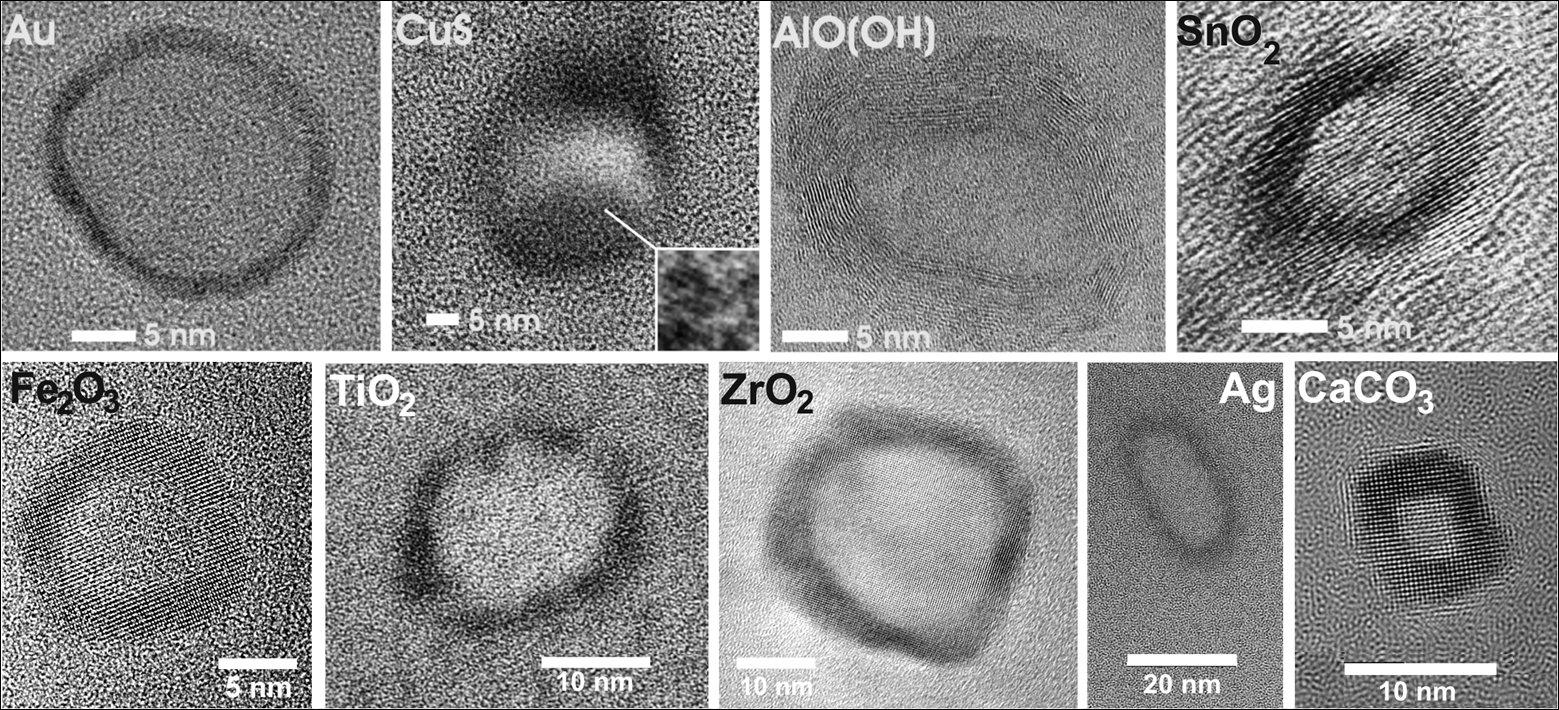
Figure 1. Various hollow nanospheres made via microemulsion synthesis (Adv. Mater. 2009, 21, 1586–1590).
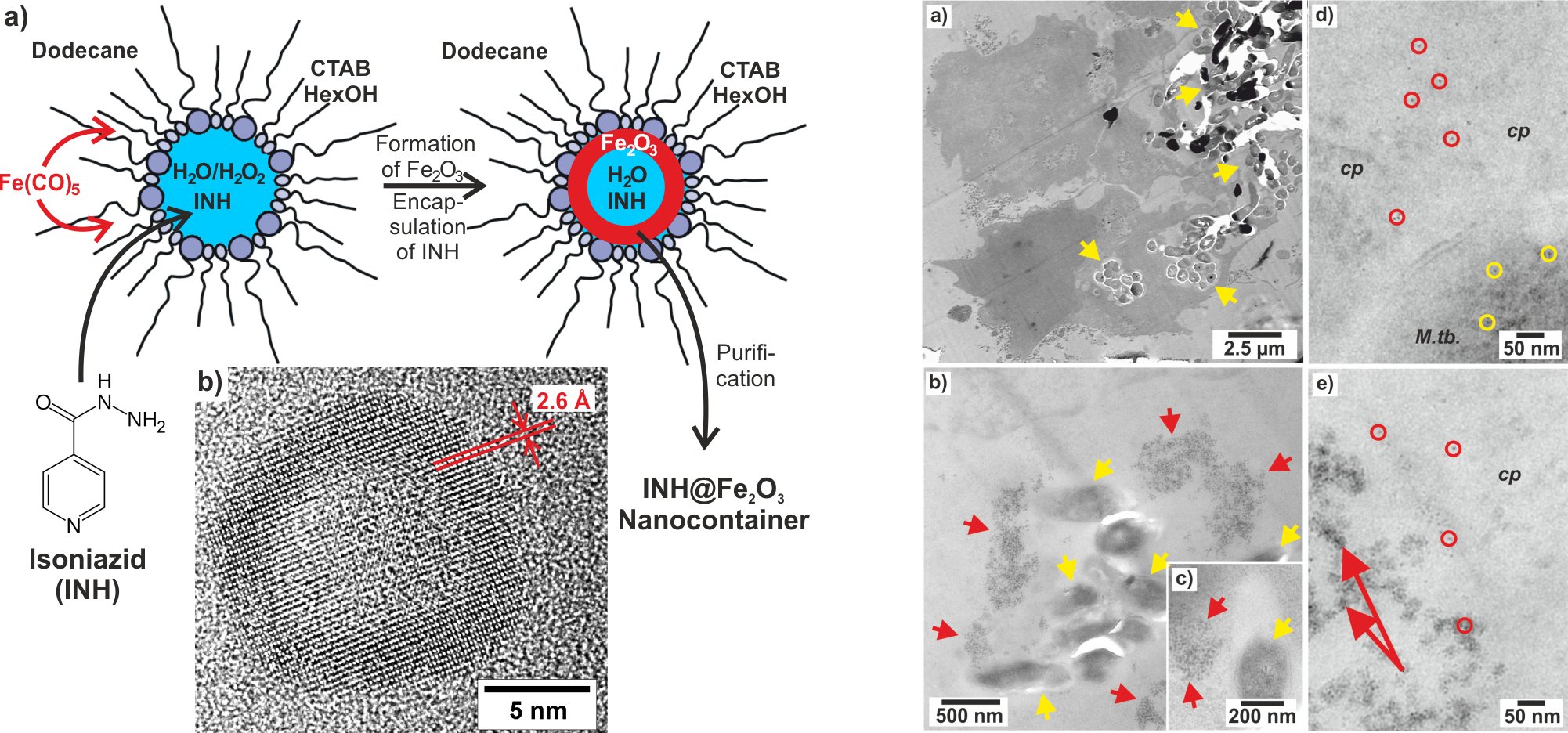
Figure 2. INH@Fe2O3 hollow nanospheres containing antibiotic isoniacide (INH) for tuberculosis therapy (Angew. Chem. Int. Ed. 2015, 54, 12597–12601).
Figure 3. TiO2@CeO2-Pt hollow nanospheres made via NaCl templates for oxidation catalysis (Chem. Cat. Chem. 2024, accepted; .Nanoscale 2021, 13, 2005-2011).
For more information see:
V. Rein, E. Zittel, K. Hagens, N. Redinger, U. Schepers, H. Mehlhorn, U. Schaible, C. Feldmann*, Zirconyl Hydrogenphosphate Nanocontainers for Flexible Transport and Release of Lipophilic Cytostatics, Insecticides and Antibiotics. Adv. Funct. Mater. 2019, 29, 1900543(1-11).
S. Wolf, C. Feldmann*, Microemulsions: Options to Expand the Synthesis of Inorganic Nanoparticles (Review). Angew. Chem. 2016, 128, 15958–15984; Angew. Chem. Int. Ed. 2016, 55, 15728–15752.
P. Leidinger, J. Treptow, K. Hagens, J. Eich, N. Zehethofer, D. Schwudke, W. Öhlmann, H. Lünsdorf, O. Goldmann, U. E. Schaible*, K. E. J. Dittmar,* C. Feldmann*, Isoniazid@Fe2O3 Nanocontainers and Their Antibacterial Effect on Tuberculosis Mycobacteria. Angew. Chem. Int. Ed. 2015, 54, 12597–12601.
H. Gröger, F. Gyger, P. Leidinger, C. Zurmühl, C. Feldmann*, Microemulsion Approach to Nanocontainers and its Variability in Composition and Load. Adv. Mater. 2009, 21, 1586–1590.
Inorganic-organic hybrid nanoparticles (IOH-NPs)
Inorganic-organic hybrid nanoparticles (IOH-NPs) represent a novel class of materials for biomedical application including multimodal imaging and drug release. The material concept relates to a platform of compounds with > 50 different IOH-NPs and drugs by now. The concept was originally developed by us and filed with several international patents.
The saline IOH-NPs with a general composition [ZrO]2+[RfunctionOPO3]2- or [GdO]+[RfunctionSO3]- consist of an inorganic cation (i.e., [ZrO]2+, [GdO]+) and a functional organic anion [RFunctionOPO3]2- or [RFunctionSO3]- (RFunction: functional organic group). The essential role of the inorganic cation is to guarantee the insolubility of the IOH-NP in water. IOH-NPs exhibit an unprecedented high dye and/or drug load (i.e. 70-85 wt-% of total IOH-NP weight). Specific examples of IOH-NPs are [ZrO]2+[MFP]2-, [GdO]+[AMA]–, or [GdO]+[ICG]– that show green, red or infrared emission (MFP: methylfluorescein phosphate; AMA: amaranth red; ICG: indocyanine green) (Figure 1).
The material concept of the IOH-NP can be further expanded to drug delivery and drug release including [ZrO]2+[BMP]2-, [ZrO]2+[FdUMP]2-, and [ZrO]2+[CLP]2- with the anti-inflammatory agent betamethason phosphate (BMP), the cytostatic agent 5’-fluoro-2’-deoxyuridine 5’-monophosphate (FdUMP) or the antibiotic clindamycinephosphate (CLP). In vitro and in vivo studies confirm excellent activity and high biocompatibility. [ZrO]2+[BMP]2- shows an effective inflammatory response in vitro and in vivo (Figure 2). Recently, [ZrO]2+[GMP]2- IOH-NPs (GMP: gemcitabine monophosphate) turned out as most promising to treat pancreatic ductal adenocarcinoma (PDAC) (Figure 3). Specific advantages are the very high drug load (76% GMP of total IOH-NP mass); high uptake and high activity in/on tumor, high activity also on metastasis, low side effects, by-pass of gemcitabine-based resistances.
Specific advantages of IOH-NPs are:
- Aqueous synthesis, high drug load (50-90 % of total nanoparticle mass)
- Platform concept with high flexibility regarding the type of drug
- Use for multimodal imaging
- High activity at low side effects
- Application in medicine to treat cancer, inflammation, infection
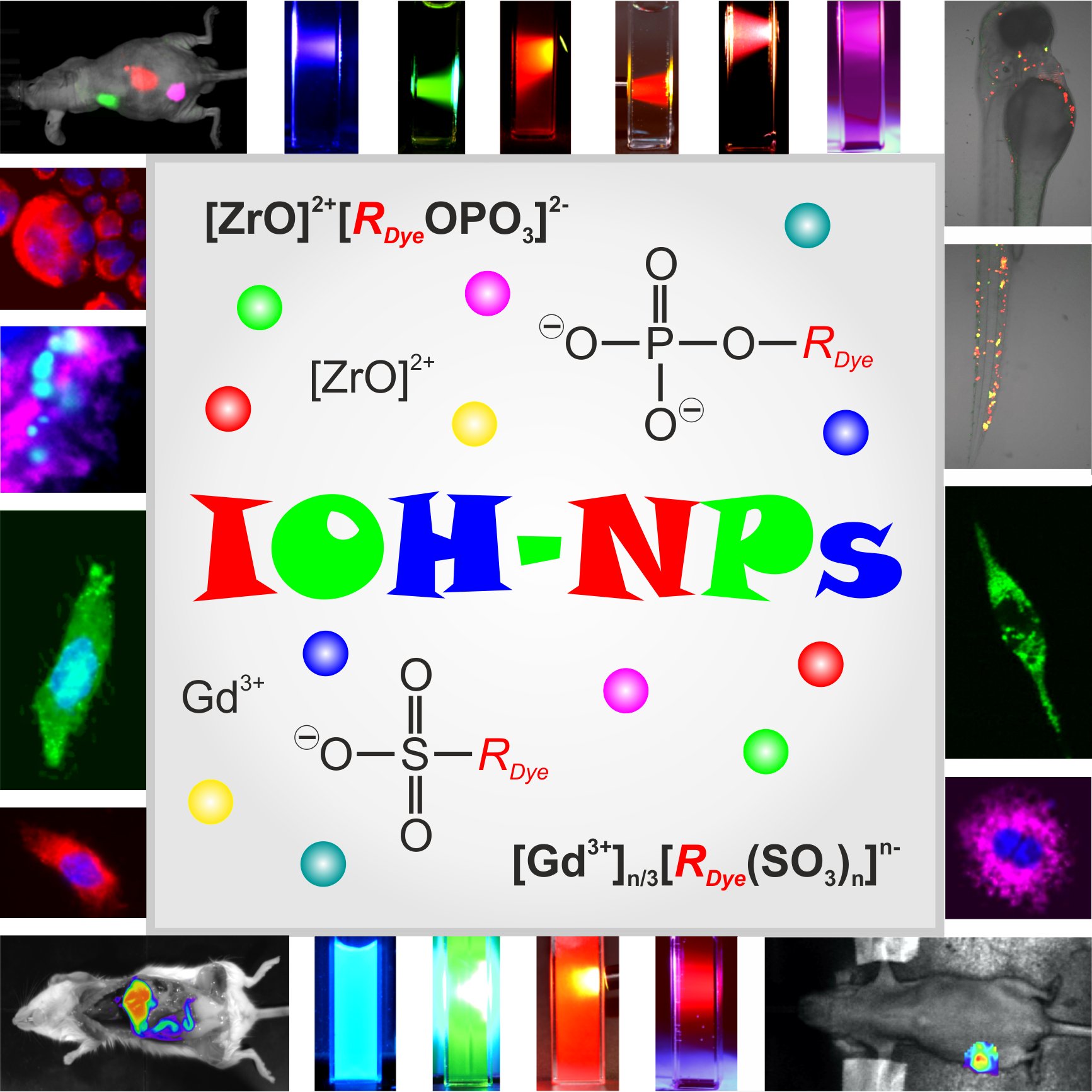
Figure 1: Fluorescence of different IOH-NPs (review: ChemNanoMat 2019, 5, 24-45).
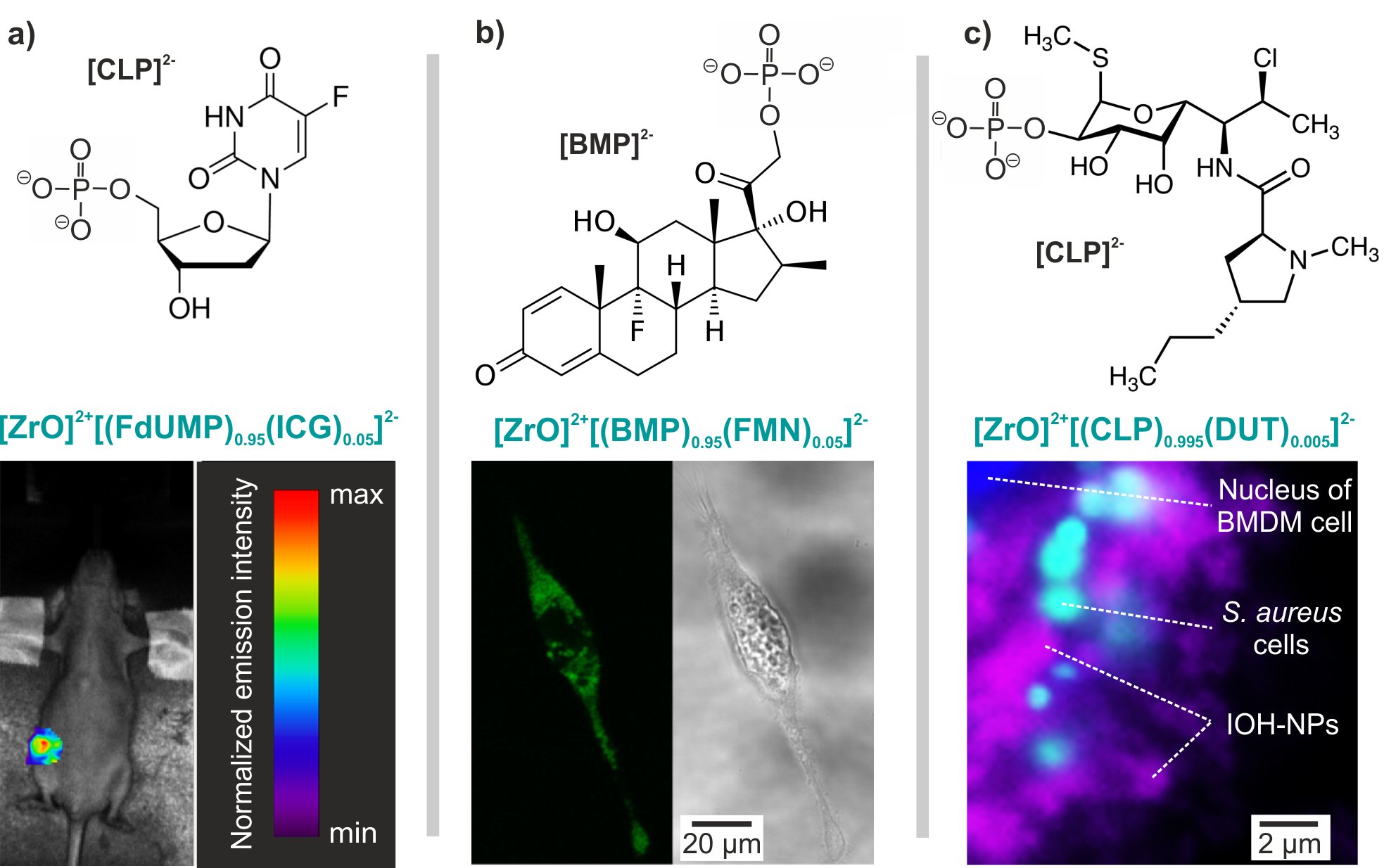
Figure 2: Drug delivery with IOH-NPs (review: ChemNanoMat 2019, 5, 24-45).
Figure 3: [ZrO]2+[GMP]2- IOH-NPs (GMP: gemcitabine monophosphate) to treat pancreatic ductal adenocarcinoma (PDAC) (Adv. Mater. 2023, 2305151).
For more information see:
M. Ischyropoulou, K. Sabljo, L Schneider, C. M. Niemeyer, J. Napp*, C. Feldmann*, F. Alves*High-Load Gemcitabine Inorganic-Organic Hybrid Nanoparticles as Image-Guided Tumor-Selective Drug-Delivery System To Treat Pancreatic Cancer. Adv. Mater. 2023, 2305151(1-15)
D. Rudolph, N. Redinger, K. Schwarz, F. Li, G. Hädrich, M. Cohrs, L. A. Dailey,* U. E. Schaible*, C. Feldmann*, Amorphous Drug Nanoparticles for Inhalation Therapy of Multidrug-Resistant Tuberculosis. ACS Nano 2023, 17, 9478-9486.
M. Khorenko, A. Meschkov, J. Napp, J. Pfeifer, J. Stier, F. Alves*, U. Schepers*, C. Feldmann*, Theranostic Inorganic-Organic Hybrid Nanoparticles with a Cocktail of Chemotherapeutic and Cytostatic Drugs. J. Mater. Chem. B 2023, 11, 3635-3649.
M. Poß, E. Zittel, C. Seidl, A. Meschkov, L. Muñoz, U. Schepers,* C. Feldmann*, Gd43+[AlPCS4]34–: Multi-functional Nanoagent Generating 1O2 for Photodynamic Therapy. Adv. Funct. Mater. 2018, 28, 1801074(1-8).
J. G. Heck, J. Napp, S. Simonato, J. Möllmer, M. Lange, H. R. Reichardt, R. Staudt, F. Alves,* C. Feldmann*, Multifunctional Phosphate-based Inorganic-Organic Hybrid Nanoparticles. J. Am. Chem. Soc. 2015, 137, 7329−7336.
Oxides with Specific Morphology for Catalysis & Photocatalysis
The exploration of efficient daylight-activated photocatalysts remains a most essential challenge. Here, anatas-TiO2 is most widely applied but due to it wide band gap (2.8 eV) only active under UV-light excitation. Yet known daylight-activated photocatalysts often contain toxic and/or expensive constituents (e.g., CdS, BiVO4).
Our activities related to catalysis and photocatalysis address metal tungstates, metal molybdates, metal vanadates, metal niobates and metal tantalates in regard of the following aspects:
- Realization of crystalline nanostructures via liquid-phase synthesis
- Morphology control
- Evaluation of properties including color, catalysis, photocatalysis, conductivity
- Daylight-activated catalysis
For the first time, we could present β-SnWO4 as a new photocatalyst. This compound is as elusive as promising. Thermodynamically favored above 670 °C, but metastable at room temperature, the synthesis of crystalline and nanoscaled β-SnWO4 at room temperature is a challenge. Via precise control of the experimental conditions, we could prepare high-quality nanoparticles as well as facetted microcrystals of β-SnWO4 including dodecahedra, cubes, and hierarchically multiarmed architectures entitled as spikecubes (Figure 1). β-SnWO4 nanoparticles turned out as highly promising for photodynamic therapy (PDT). Thus, reiterated 5-minutes illumination with a blue-light LED is sufficient to induce high cytotoxicity.
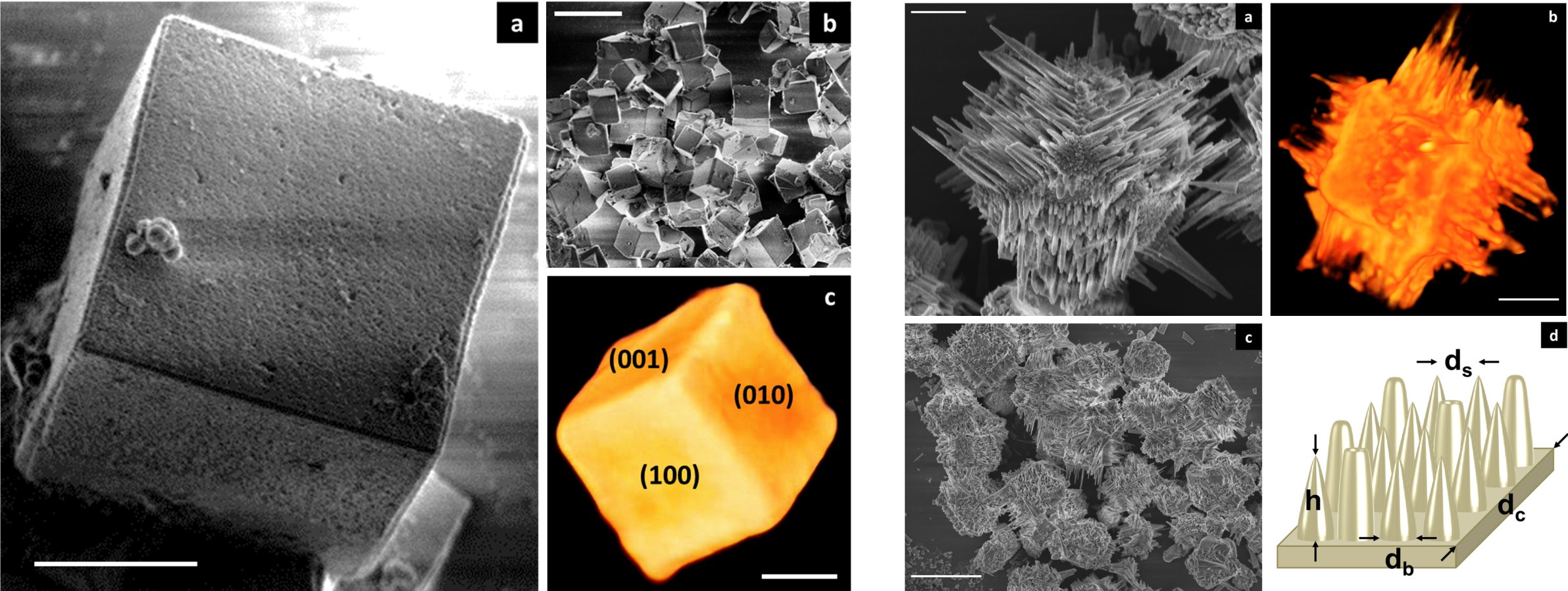
Figure 1. β-SnWO4 photocatalyst with specific morphology: dodecahedra, cubes, spikecubes (ACS Catal. 2016, 6, 2357–2367).
As a highly promising photocatalyst with peculiar composition and structure we could recently prepare Au@Nb@HxK1-xNbO3 nanopeapods (Figure 2). This structure emulates the growth pattern of natural plant: peapods. Thus, core-shell Au@Nb nanoparticles (the nanopeas) are seeding in the cavity of semiconducting HxK1-xNbO3 nanoscrolls (the nanopods). This unique structure promotes near-field plasmon-plasmon coupling between bimetallic Au@Nb nanoantennas, endowing the UV-active HxK1-xNbO3 semiconductor with strong VIS and NIR light harvesting abilities to trigger dye photodegradation and water photoelectrolysis.
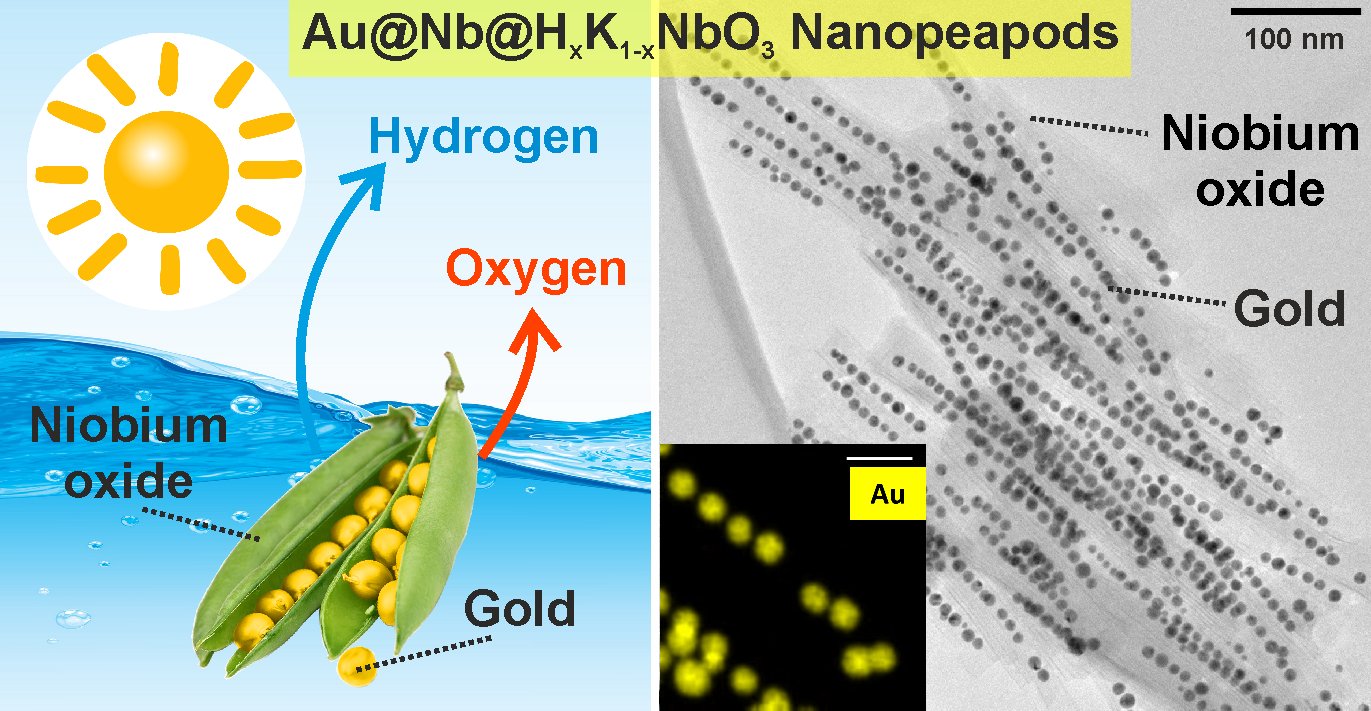
Figure 2. Au@Nb@HxK1-xNbO3 nanopeapods with core-shell Au@Nb nanoparticles as nanopeas located in HxK1-xNbO3 nanoscrolls as nanopods (Nature Commun. 2018, 9, 232(1–11)).
For more information see:
Y.-C. Chen, Y.-K. Hsu, R. Popescu, D. Gerthsen, Y.-G. Lin, C. Feldmann*, Au@Nb@HxK1-xNbO3 Nanopeapods with Near-infrared Active Plasmonic Hot-Electron Injection for Water Splitting. Nature Commun. 2018, 9, 232(1–11).
Y.-C. Chen, Y.-G. Lin,* L.-C. Hsu, A. Tarasov, P.-T. Chen, M. Hayashi, J. Ungelenk, Y.-K. Hsu,* C. Feldmann*, Tungstate Nanoparticles: A Photosensitizer for Photodynamic Tumor Therapy. β-SnWO4 Photocatalyst with Controlled Morphological Transition of Cubes to Spikecubes. ACS Catal. 2016, 6, 2357–2367.
C. Seidl, J. Ungelenk, E. Zittel, T. Bergfeldt, J. P. Sleeman, U. Schepers*, C. Feldmann*, Tin Tungstate Nanoparticles: A Photosensitizer for Photodynamic Tumor Therapy. ACS Nano 2016, 10, 3149–3157.
Ionic liquids in Inorganic-Synthesis Chemistry
Ionic liquids in Inorganic-Synthesis Chemistry
Our activities related to ionic liquids address the question: What is special about ionic liquids in inorganic synthesis? As there are various syntheses strategies available in inorganic chemistry, the question regarding the specific advantage of ionic liquids is even more interesting. Generally, ionic liquids (ILs) are known for their unusual properties (e.g., wide liquid range, excellent thermal stability, wide electrochemical window, weakly coordinating constituents). For our studies, two aspects are most interesting: the weakly coordinating properties and the wide electrochemical window of ionic liquids. In principle, selected ionic liquids can make it possible to work with elemental fluorine (E0 = +3.1 V) and with elemental caesium (E0 = -2.9 V), without the solvent decomposing! Based on these conditions, we address reactions with highly oxidizing starting materials (i.e. halogens) as well as with highly reducing starting materials (i.e. carbonyl metals) in ionic liquids.
This is illustrated in the following examples: a) The carbonyl cluster compound [GeRu6(CO)18HI] with unique structure and bonding including a GeRu6 cluster core, a planar GeRu4HI unit, extensive multi-center bonding, and an aromatic ring current similar to benzene (9-10 nA T−1) (Figure 1); b) the polybromide [C4MPyr+]2[(Br–)2×9(Br2)] representing the first three-dimensional polybromide network (Figure 2); c) the crown-ether coordination compounds ZnX2(18-crown-6), EuX2(18-crown-6) (X: Cl, Br, I), MnI2(18-crown-6), Mn3Cl6(18-crown-6)2, Mn3I6(18-crown-6)2 and Mn2I4(18-crown-6), which show unique luminesence properties with outstanding quantum yields up to 100% as well as nonlinear optical (NLO) effects (Figure 3).
Figure 1: Carbonyl cluster compound [GeRu6(CO)18HI] with extensive multi-center bonding and benzene-like aromatic ring current (Adv. Sci. 2024, accepted).
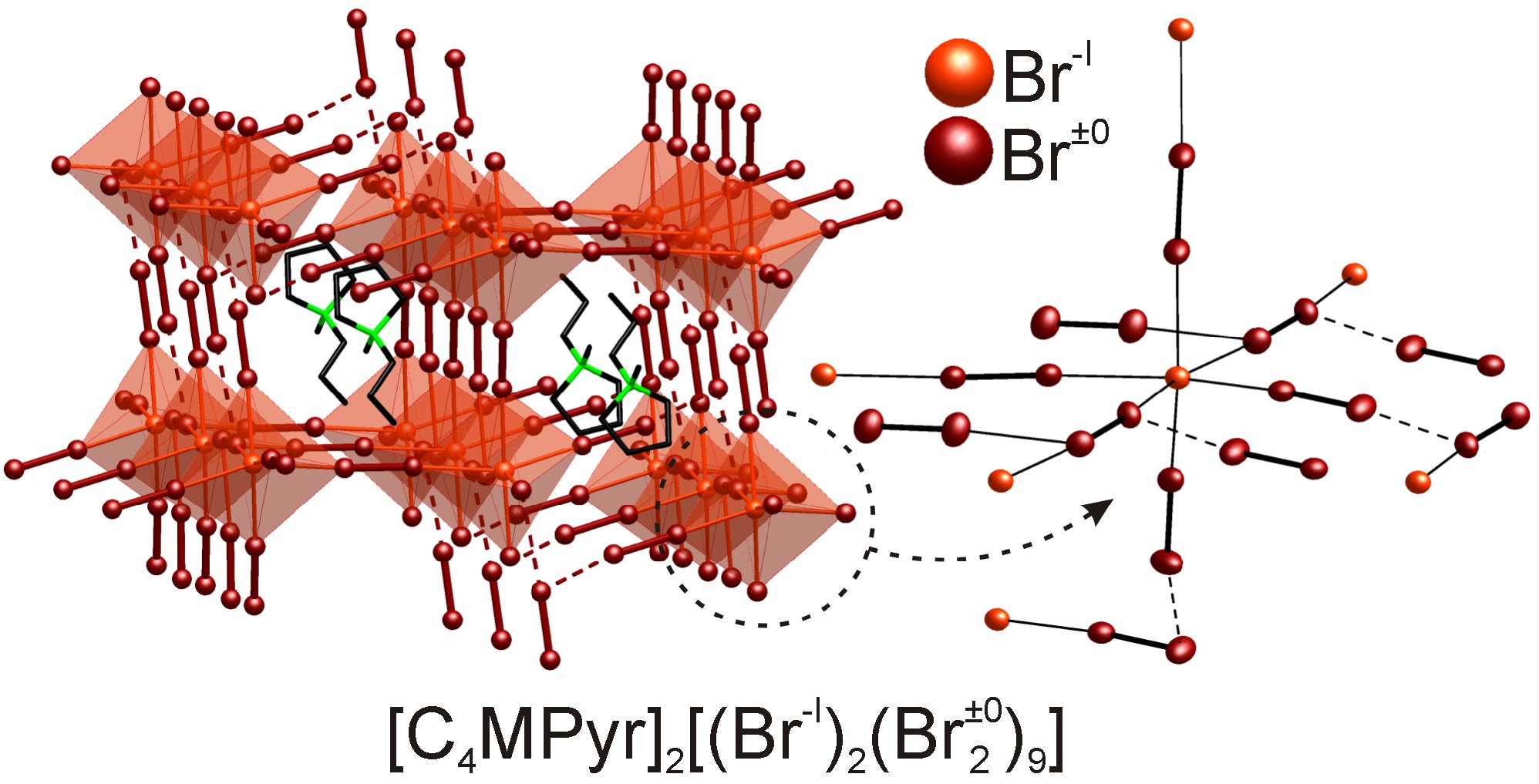
Figure 2: [C4MPyr]2[Br20] with [(Br–)2(Br2)9] network composed of central bromide anions (light red) that are interlinked via molecular bromine (dark red) (Angew. Chem. Int. Ed. 2011, 50, 4970–4973).
Figure 3: Unique luminescence properties of crown-ether coordination complexes (J. Am. Chem. Soc. 2021, 143, 798–804).
For more information see:
S. Wolf, R. Köppe, J. Treptow, W. Feuerstein, J. Wenzel, F. Breher, P. W. Roesky, F. Weigend, W. Klopper, C. Feldmann*, [GeRu6(CO)18HI]: A Germanium-Centered Ruthenium Carbonyl Cluster with Aromatic Ring Current. Adv. Sci. 2024, accepted.
E. Merzlyakova, S. Wolf, S. Lebedkin, L. Bayarjargal, B. L. Neumeier, D. Bartenbach, C. Holzer, W. Klopper, B. Winkler, M. Kappes, C. Feldmann*, 18-Crown-6 Coordinated Metal Halides with Bright Luminescence and Nonlinear Optical Effects. J. Am. Chem. Soc. 2021, 143, 798–804.
S. Wolf, R. Köppe, T. Block, R. Pöttgen, P. W. Roesky, C. Feldmann*, [SnI8{Fe(CO)4}4]2+: Highly-coordinated Sn+III8 Subunit with Fragile Carbonyl Clips. Angew. Chem. Int. Ed. 2020, 59, 5510–5514.
M. Wolff, J. Meyer, C. Feldmann*, [C4MPyr]2[Br20] − Ionic Liquid based Synthesis of the first three-dimensional Polybromide Network. Angew. Chem. Int. Ed. 2011, 50, 4970–4973.

