
Breher Research Group BlueSky Account (@brehergroup.bsky.social)
MEHR
Homepage of the KIT
MEHRRecent News
Stabilities and Limitations in the Reactivity of Al/C and Ga/C FLPs: A detailed study on the reactivity of ambiphilic metallacycles is presented. In a combined computational and experimental study, the unexpected reactivity towards isocyanides as well as stability and reactivity limits of the title compounds is described, including their reactivity towards water and methanol. Interested? Follow the link.
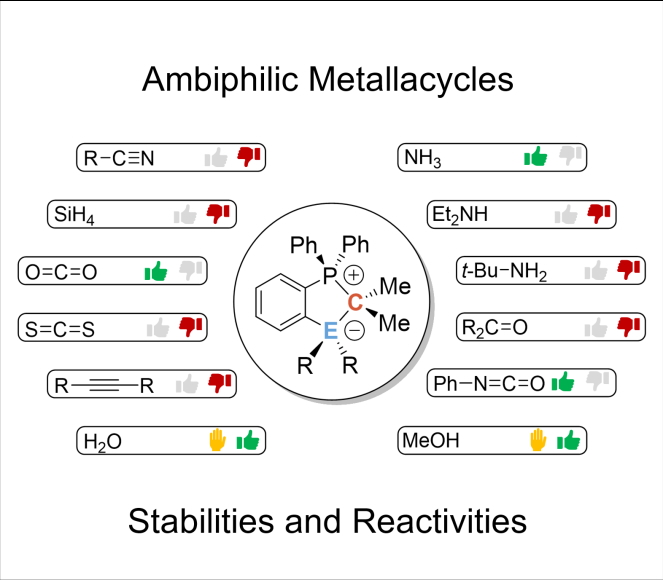
A new Al/P FLP: The first mono ortho-phenylene bridged Al/P Frustrated Lewis Pair was synthesized and characterized. Its reactivity was probed against several small molecules. Furthermore, suitable entry point for generating other Al/C-based FLPs starting from the Al/P-based title compound was identified. Have a look at the recent paper: link.
Cover Picture: The chelating ligand of one of the title complexes shown on the cover picture is coordinated by three nitrogen donors (blue) to the central aluminum atom (wine-red). The latter consists of two more substituents, i.e. two phenyl rings. One of these phenyl rings is “blown” away by the wave of light coming from the right of the picture. The homolytic bond rupture event is highlighted and the phenyl ring flying away is shown in red due to its “hot”, i.e. radical nature.

Visible-Light Activation of Al(III) Complexes and their Radical Reactivity: We report on the synthesis and characterization of a series of (mostly) air-stable diorganyl bis(pyridylimino) isoindolide (BPI) aluminum complexes and their chemistry upon visible-light excitation. The redox non-innocent BPI pincer ligand allows for efficient charge transfer homolytic processes of the title compounds. This makes them a universal platform for the generation of carbon-centered radicals. The photo-induced homolytic cleavage of the Al−C bonds was investigated by means of stationary and transient UV/Vis spectroscopy, spin trapping experiments, as well as EPR and NMR spectroscopy. The experimental findings were supported by quantum chemical calculations. Reactivity studies enabled the utilization of the aluminum complexes as reactants in tin-free Giese-type reactions and carbonyl alkylations under ambient conditions, which both indicated radical-polar crossover behavior. A deeper understanding of the physical fundamentals and photochemical process was provided, furnishing in turn a new strategy to control the reactivity of bench-stable aluminum organometallics. The paper was published in Angewandte Chemie. Many thanks to all authors of the paper for this great collaborative study! (link).

Quo Vadis CO2 Activation: The herein reported aluminum and gallium/carbon-based ambiphiles, a new class of hidden frustrated Lewis pairs consisting of a phosphorus ylide featuring an aluminum or gallium fragment in the ortho position of a phenyl ring scaffold, react stoichiometrically with CO2 forming the stable adducts but also act as an efficient catalyst for the reduction of CO2 mediated by HBpin. Many thanks to all authors of the paper for this great collaborative study published in Chem. Eur. J. (link).

Nice little story: We herein report the isolation and full characterization of the previously unknown polymer solvent-free lithium alanate {Li[t-Bu2AlH2]}n (1), which was isolated in pure form from the long-known reaction of AlBr3 with three equivalents of t-BuLi. Published in Eur. J. Inorg. Chem. (link).

Reactive Tetryl Radicals: Herein, we present that the radicals [Ph3PC(Me)EMes2]˙ (2Si and 2Ge) can be generated from the α-silylated and α-germylated phosphorus ylides Ph3PC(Me)E(Cl)Mes2 (1Si and 1Ge) through one-electron reduction with Jones’ dimer (MesNacNacMg)2 in benzene. Although isolation of the free radicals was not possible, the products of the intramolecular addition of the radicals to a phenyl substituent of the phosphorus moiety, followed by subsequent reaction with 2Si or 2Ge to the isolated species 3Si and 3Ge, respectively, were observed. This transformation witnesses a dearomative 1,4-addition of tetryl radical species to the phenyl scaffold in a stereoselective anti-fashion. This work recently appeared in Dalton Trans. (link).

Switchable Lewis Acids #2: Another nice collaborative study with the Paradies group on ferrocenyl boranes and their application as Lewis acids in epoxide rearrangements, accepted for publication in Eur. J. Inorg. Chem. Many thanks to all involved! (link).

A crystalline aluminium–carbon-based ambiphile capable of activation and catalytic transfer of ammonia in non-aqueous media: Despite recent achievements in the field of frustrated Lewis pairs (FLPs) for small molecule activations, the reversible activation and catalytic transformation of N–H-activated ammonia remain a challenge. Here we report on a rare combination of an aluminium Lewis acid and a carbon Lewis base. A so-called hidden FLP consisting of a phosphorus ylide featuring an aluminium fragment in the ortho position of a phenyl ring scaffold is introduced. Although the formation of the Lewis acid/base adduct is observed in the solid state, which at first glance leads to formally quenched FLP reactivity, we show that the title compound readily reacts with non-aqueous ammonia thermoneutrally and splits the N–H bond reversibly at ambient temperature. In addition, NH3 transfer reactions mediated by a main-group catalyst are presented. This proof-of-principle study is expected to initiate further activities in utilizing N–H-activated ammonia as a readily available, atom-economical nitrogen source.
The paper has been published in Nature Chemistry (link). Many thanks to all authors of the paper for this great collaborative study!
See also the press release of the KIT (link) and the nice "behind the paper" blog post of Felix Krämer (link)!
Mentions in news and blogs: Altmetric.

Recent Review on heteroatom-centered diradical(oid)s, which have been in the focus of molecular main group chemistry for nearly 30 years. During this time, the diradical concept has evolved and the focus has shifted to the rational design of diradical(oid)s for specific applications. This review article begins with some important theoretical considerations of the diradical and tetraradical concept. Based on these theoretical considerations, the design of diradical(oid)s in terms of ligand choice, steric, symmetry, electronic situation, element choice, and reactivity is highlighted with examples. The application of diradical(oid)s, for example in small molecule activation or as molecular switches, is also highlighted. The final part begins with application-related details of the spectroscopy of diradical(oid)s, followed by an update of the heteroatom-centered diradical(oid)s and tetraradical(oid)s published in the last 10 years since 2013.
The review has been published in Chem. Rev. (link). Many thanks to all authors of the paper for this great collaborative study!

The rare earth element complexes (Ln=Y, La, Sm, Lu, Ce) of several κ6N-coordinating tripodal ligands have been synthetized and characterized. After estimation of their donor capabilities, the ligands selectivity through the lanthanide cations have been investigated. (link).

Non-innocent ligands: This work reports on the synthesis and characterization of alkali metal complexes of BPI and Me2BPI in reduced oxidation states. CV, EPR and XRD studies revealed detailed information about the unknown lower oxidation states of BPI, which is well-established in coordination chemistry but so far only in its redox-neutral state. (link). This article is part of the EurJIC special collection "Evolving with Inorganic Chemistry for 25 Years".

One-electron oxidation converts the ferrocene-substituted borane into a Lewis superacid, as reported in a recent collaborative study with the Paradies group (link). The Lewis superacid activates almost inert carbon–fluorine and sulfur–fluorine bonds for reductions, alkylations, and arylations to form new chemical bonds.

Enjoying the sun: The cover illustrates the potential of heterobimetallic AuI/RuII complexes to facilitate the P arylation of H-phosphonates with diazonium salts, while visible light kicks off the dual gold and photoredox catalytic cycle. The underlying structure of the catalysts’ coordinating ligand system is accessible by a novel postfunctionalization approach, and the stereochemistry can be controlled by the order of metal coordination and click reaction. More information can be found in the recent Chem. Eur. J. paper (DOI: 10.1002/chem.202201856).

L- and Z-type ligands side by side: The overall neutral α-borylated phosphorus ylide Ph3PC(Me)BEt2 behaves like a polar borataalkene and can act as acyclic, ambiphilic π-type ligand with L- and Z-type functionalities side by side. In the complexes [MX{η2-Ph3PC(Me)BEt2}] (M = Cu, (Ag), Au; X = Cl, NTf2), the bonding is dominated by the highly nucleophilic ylidic carbon atom (L-type ligand). The Lewis acidic boron atom furnishes nonetheless a small but significant bonding contribution (Z-type ligand). Great study together with the Fernández group published in Chem. Commun. (link).

Collaborative study 1: It was a great pleasure to support the Paradies group with EPR measurements on fascinating small-band gap quinoid thioacene derivatives. Have a look at this recently accepted collaborative research paper published in Chem. Eur. J. (link).

Collaborative study 2: In a recent collaborative study with the Podlech group we examinded an interesting cyclopenta-fused polyaromatic hydrocarbon. It turned out that this radical is best described with its radical centre being in the outer five-membered rings, which allows for the largest number of fully intact benzene rings. Its triradical character is small but not neglectable (link).

FcBipy: Novel redox-active bipyridine ligands based on 1,1’ difunctionalized ferrocenyl backbone, in conjunction with their metal complexes, are reported. The influence of the implemented functional groups on both the iron-centred redox potential and the N,N’-coordinated nickel complexes were confirmed for the reductive elimination reaction of an aryl ether induced by oxidation of the corresponding methoxides. Nice collaborative paper on multimetallic complexes (link).

A warm welcome to Christina Ruiz-Martínez, guest scientist from the Fernández group in Almería (link)

Cover: Happy to share our cover artwork on our recent paper on „Accessing Cationic α‐Silylated and α‐Germylated Phosphorus Ylides“. Many thanks to Melina Dilanas and all other contributors!

Competition rules! α-Silylated and -germylated phosphorus ylides of the general formula R3PC(R1)E(Cl)R22 bearing one chloride substituent on the group 14 element were synthesized and fully characterized. Chloride abstraction reactions provided access to dimeric or monomeric cations featuring a high fluoride ion affinity despite the relatively strong CYlide–E interaction with high double bond character. The paper has just been accepted for publication in Chem. Eur. J. (link). Congratulations to all contributing co-workers!

Nice Cover designed by Bernhard Birenheide. Congratulations!! A retro-style arcade machine needs an oxidant or a proton to be inserted to start playing and to switch the donating capabilities of novel phosphine ligands orthogonally and reversibly. Rh-catalysed hydrosilylation showed that these triggers can influence either the rate of conversion and/or the product distribution. The Japanese kanji for “iron” highlights the ferrocene moiety in the ligands′ metal complexes. (link).

Group excursion! We had a super funny and enjoyable group excursion to the Waldseilpark in Durlach, also including good food and some refreshments afterwards. Thanks to all group members for the wonderful day!!

"Double switch" In a recent collaborative study with the Dielmann group (Innsbruck) we have published a communication in Chem. Eur. J.: Novel multistimuli-responsive phosphine ligands comprising a redox-active [3]dioxaphosphaferrocenophane backbone and a P-bound imidazolin-2-ylidenamino entity that allows switching by protonation are reported. Investigation of the corresponding metal complexes and their redox behaviour are reported and show the sensitivity of the system towards protonation and metal coordination. The experimental findings are supported by DFT calculations. Protonation and oxidation events are applied in Rh-catalysed hydrosilylations and demonstrate a remarkable influence on reactivity and/or selectivity. (link).

High NON! Different ways for the synthesis of NON-ligated N-heterocy- clic germylene (3), stannylene (4) and plumbylene (5) were carried out. The title compounds were characterized using standard methods including X- Ray diffraction. Initial investigations on the coordination behavior of 3–5 towards [Rh(CO)2Cl]2, [W(CO)6] and [Ni(CO)4] were performed. The σ- donor and π-acceptor properties were evaluated using density functional theory. The results have been published recently and were dedicated to Prof. Hansgeorg Schnöckel on the occasion of his 80th birthday (link).

Cooperative Effects in Multimetallic Complexes applied in Catalysis: In a recent collaborative study with the Sarkar group (Stuttgart) we have published a review article in ChemCatChem. The field of multimetallic catalysis is rapidly developing and some multimetallic complexes catalyse organic transformations to yield the desired products in more efficient ways owing to the combined action of different metals in a cooperative fashion. This concept article describes the recent advances of cooperative catalysis playing in multimetallic systems such as homo‐multimetallic complexes with short metal‐metal distances, homo‐multimetallic complexes with long metal‐metal distances, hetero‐multimetallic complexes and metallocene‐based multimetallic complexes with special attention towards redox‐switchable catalysis. Examples are illustrated in which the use of multimetallic complexes show clear enhancement of catalytic outcomes when compared with the sum of their corresponding mononuclear counterparts. Furthermore, several examples are discussed showing the effects of electronic communication in cooperative systems. (link).

Capture the gold(fish)!: Donor‐substituted dihalobiphenyls are suitable pre‐ligands for the preparation of luminescent, non‐palindromic [(C^C^D)AuIII] complexes with a lateral pyridine or carbene donor. Like an anglerfish's rod enmeshes its prey, the lateral donor tunes the gold‘s electronic properties which is hold by the rigid biphenyl mouth. Synthesis, spectroscopy and quantum chemistry draw a comprehensive picture about the new complexes prepared (link).

"double trouble": A highly luminescent non-palindromic [(C^C^N)Pd] pincer complex forms upon reacting pyridine-substituted 2,2′-diiodo-biphenyl with [Pd(PPh3)4]. This case study establishes for the first time that the title compound is formed via a double oxidative addition – comproportionation sequence. DFT and TDDFT calculations complement mechanistic and photophysical characterizations (link).

Chem. Sci. Inside Cover: Again, a great collaborative study with the Krossing group. The manuscript entitled “Completing the Triad: Synthesis and full Characterization of Homoleptic and Heteroleptic Carbonyl and Nitrosyl Complexes of the Group VI Metals” has just been published in Chem. Sci. (link).

Emmy Noether Fellowship for Dr. Alexander Hinz (link)! Congratulations for these great news!

Al/N-based FLPs? A series of alane-substituted imidazolyl compounds, featuring different architecture is presented. For dimeric species steric dependent formation of symmetric and an asymmetric species were observed. Reactivity studies with small molecules revealed no reactivity as hidden FLP. Nevertheless, ambiphilic behavior of the imidazolyl alanes has been observed in cases during this study, which has recently been accepted for publication (link).

Electronic Frustration: The sterically unencumbered α-borylated phosphorus ylide Me3PC(H)B(iBu)2 has been synthesized, characterized, and investigated with the aid of density functional theory calculations. The title compound has been demonstrated to react with various small molecules such as NH3, CO, CO2 and the heterocumulenes PhNCX. In part, different reaction products are found as compared to the previously reported derivative Ph3PC(Me)BEt2. For details, see the recent full-paper published in Chem. Eur. J. (link), which has been designated as “Hot Paper”.

Great canoeing tour in July 2019: not astonishingly, repeated capsizing was no problem on the hottest day ever measured in Germany…

Scorpionates meet Phosphines: In a recent Dalton Trans. we have described new phosphine-functionalised tris(pyrazolyl)methane ligands, their coordination flexibility and their ability to generate heterobimetallic complexes (link). The results have recently been highlighted in Chemistry Views (click here).

Cooperative effects in hydroamination catalysis (collaboration with Jan Paradies): Very high activities were observed in the redox-induced hydroamination of alkynes by employing a redox-active gold(I) complex featuring a phosphonite-based ligand. Very low catalyst loadings (down to 10 ppm) and very mild conditions (25°C) were used. The hydroamination proceeds roughly two-fold faster with the in-situ oxidized catalysts than with its reduced form. The results have now been published in Chem. Commun. (click here) as part of the ChemComm themed issue “Switchable Catalysis” (click here) and featured with an inside back cover.

Nature Communication published (collaboration with the Krossing group): Carbonyl Cations go Open Shell… Chromium hexacarbonyl was oxidized with the [NO]+ salt of a weakly coordinating anion. Depending on the conditions, the first 17 valence electron (VE) configurated, homoleptic carbonyl radical cation [Cr(CO)6]•+ (kinetic product), or the 18 VE species [Cr(CO)5(NO)]+, the first mixed chromium carbonyl/ nitrosyl cation, is formed (thermodynamic product). The results have now been published in Nature Communications (link). Congratulations to all co-authors!
♠ See also the press release of the University of Freiburg (link) and a “behind the paper” post by Jan Bohnenberger (link).
♠ Nature Communications Editors’ Highlights webpage on inorganic and physical chemistry (link)
♠ Selected further references: ChemistryViews (link); further links (Altmetric).

Electronic Frustration: The concept and the first example for electronic frustration within a C–B π-bond arising from the competition for a lone pair of electrons is reported. The α-borylated phosphorus ylide (α-BCP) Ph3PC(CH3)BEt2 has been synthesized, characterized, and investigated with the aid of density functional theory calculations. These show the presence of a highly polarized C–B π-bond, induced by the electron withdrawing Ph3P+ substituent. This competition for the C-based lone pair of electrons leads to an FLP-type reactivity (FLP = frustrated Lewis pair), which has been demonstrated by reacting the title compound with various small molecules such as CO, CO2, COS, CS2 or the heterocumulenes PhNCO and PhNCS. More information can be found in the recent Communication, which has been highlighted with a Frontispiece and selected as “Hot Paper” in Chem. Eur. J. (click here). The paper also belongs to the most accessed contributions to Chem. Eur. J. in 10/2018 (link).

Great group excursion to the Black Forest in August 2018: “Gertelbacher Wasserfälle”

The Cover Feature shows a strategy to modify the metal‐metal distance in dinuclear rhodium(I) complexes based on linker‐bridged bis(amidinate) ligands. As part of the collaborative research center SFB 3MET, our group aims at understanding the underlying principles governing cooperative effects in catalysis. To rationally synthesize polynuclear complexes with tuneable metal–metal distances, a series of chelating N‐donor ligands was developed, which would concomitantly have an influence on possible cooperative effects. More information can be found in the recent EurJIC Full-paper, which has been highlighted with a Cover Feature of issue 26 (click here). In addition, we have recently reported with Peter Roesky’s group on rhodium(I) and iridium(I) complexes of ferrocenyl-functionalized amidinates and bis(amidinates) (Organometallics 2019, in press).

Nice collaborative paper with the group of Ingo Krossing (Freiburg): A facile route to cationic NiI phosphine complexes starting from recently reported [Ni(cod)2][Al(ORF)4] (1, with RF = C(CF3)3) is described. [Ni(dppp)2][Al(ORF)4] (2), [Ni(dppe)2][Al(ORF)4] (3), three-coordinate [Ni(PPh3)3][Al(ORF)4] (4) and a remarkable two-coordinate NiI phosphine complex [Ni(PtBu3)2][Al(ORF)4] (5) were investigated. The results were recently published as “Hot Paper” in Chem. Eur. J. (click here).

Great group excursion in July 2017: biking, rafting, riding carousels, enjoying the time!

A dinuclear [FeRh] complex containing a redox-active [1]phosphaferrocenophane metalloligand was employed as redox-switchable rhodium catalyst in the hydrosilylation of terminal alkynes. As evidenced by activity and selectivity differences, distinct mechanisms are operating for the neutral and the cationic complex, namely the alternative Chalk–Harrod mechanism for [FeRh] and the classical variant for [FeRh]+. An “oxidatively induced reductive elimination” step was identified as the origin of the observed cooperative effect. The results were recently published as a Frontispiece-featured Communication in Chem. Eur. J. (click here).

Multidentate Silyl Ligands: A recent review summarises the use of multidentate Si-based ligand systems in transition metal coordination chemistry. These include tri- and tetradentate pincer and scorpionate-type ligands, which have been developed in recent years in order to tailor both steric and electronic properties for achieving a defined control over the reactivity of a transition metal complex. A discussion of synthetic and structural aspects of several subgroups of ligand architectures is presented (click here).

Redox-switchable catalysis: Palladium(II) complexes of two differently substituted [1]phosphaferrocenophanes FcPR (R = Mes, biaryl) and of diphenylferrocenyl phosphine Ph2PFc were applied in redox-switchable Buchwald-Hartwig cross-coupling reactions. The results were recently published as part of the themed collection „Switchable Catalysis and Related Reactions“ in Inorg. Chem. Front. (click here).

The redox-switchable germylene [Fc(NMes)2Ge] based on a [3]ferrocenophane ligand arrangement, alongside some transition metal complexes, are reported. The change in the ligating properties upon oxidation were elucidated by DFT calculations on neutral and cationic bimetallic model complexes. The results were recently published in Chem. Eur. J. (click here).

Congratulations to Michael and Wolfram for their Procter & Gamble awards for the best MA-theses in 2015/2016! The picture taken at the ceremony on November 10 shows the recipients alongside the P&G representative and the MA-theses supervisors.

Great group excursion to the Black Forest in July 2016: “Flussbettwanderung im Murgtal”… somewhat slippery when wet

Our collaborative study with the group of Rodrigue Lescouëzec (see below) was recently featured on the front-cover of issue 8 (see also here).

Collaborative study within a Co-tutelle framework between the KIT and Sorbonne Universités, UPMC (Prof. Lescouëzec): A straightforward access to a new cyanide-bridged {Fe4Co4} “molecular box” containing a potassium ion, namely K@{[FeII(Tp)(CN)3]4[CoIII(pzTp)]3[CoII(pzTp)]} (1) (with Tp and pzTp = tris- and tetrakis(pyrazolyl)borate, respectively), is provided alongside its full characterisation. A detailed analysis of the molecular structure (X-ray diffraction, mass spectrometry, NMR spectroscopy) and electronic properties (EPR spectroscopy, SQUID magnetometry, UV/Vis spectroscopy, cyclic voltammetry) reveals that 1 shows slow magnetic relaxation and a remarkable photomagnetic effect at low temperature, which is reminiscent to some FeCo Prussian Blue Analogues (PBAs) and ascribed to a photo-induced electron transfer. However, in contrast with these inorganic polymers, the overall neutral compound 1 is soluble and remarkably stable in organic solvents such as CH2Cl2. Moreover, 1 shows an interesting redox versatility, electrochemical experiments revealing the possible access to six stable redox states. The results were recently published in Chem. Sci. (click here).

Hidden FLPs: Two aluminium diphosphamethanide complexes are reported featuring four-membered PCPAl core structures and diphosphaallyl ligands. The aluminium complex [Al(tBu)2{Mes*PCHPMes*}] reacts with molecular dihydrogen at room temperature under formation of the acyclic diphosphine Mes*PCHP(H)Mes* (PH) and the corresponding dialkyl aluminium hydride [tBu2AlH]3 (AlH, see Figure). The results were recently published as Communication in Chem. Eur. J. (click here).

Fluorinated at boron: The mono- and dinuclear copper complexes [CuII(FTp*)2] and [CuI2(FTp*)2] containing the so-far unprecedented boron-fluorinated FTp* ligand are reported. The short Cu…Cu distance in [CuI2(FTp*)2] was predicted by DFT-D3 calculations to be dictated by dispersion interactions between all atoms in the complex while the Cu-Cu dispersion contribution itself is only very small. The results were recently published in Chem. Eur. J. (click here).

Collaborative study with Gerald Hörner (TU Berlin): We have provided a straightforward access is provided to iron(II) complexes showing exceedingly slow spin-state interconversion by utilizing trigonal-prismatic directing ligands of the extended-tripod type. A detailed analysis of the interrelations between complex structure (X-ray diffraction, density functional theory) and electronic character (SQUID magnetometry, Mössbauer spectroscopy, UV/vis spectroscopy) of the iron(II) center in mononuclear complexes [Fe(L)] reveals spin crossover to occur along a coupled breathing/torsion reaction coordinate, shuttling the complex between the octahedral low-spin state and the trigonal-prismatic high-spin state along Bailar’s trigonal twist pathway. We associate both the long spin-state lifetimes in the millisecond domain close to room temperature and the substantial barriers against thermal scrambling with stereochemical constraints. In particular, the topology of the κ6N ligands controls the temporary and structural dynamics during spin crossover. The results were recently published in Inorg. Chem. (click here).

Collaborative study with the group of Wim Klopper on cooperative effects (SFB 3MET): A new concept has been applied to the analysis of UV/Vis spectra of homotrinuclear transition-metal complexes by means of a many-body expansion of the change in the spectrum induced by replacing each of the three transition-metal ions in the complex by another transition-metal ion to yield a new homotrinuclear transition-metal complex. The results were recently published in Chem. Phys. Chem. (click here) and selected as VIP paper (see also here).

Two successful PhD defenses in February and April: congratulations to Alexander Feyrer and Eric Moos!!

Group excursion to the Fun Forest Kandel in August 2015.

Collaborative study with the group of Guy Bertrand: The chemical reduction of a pyridyl-substituted cyclic (alkyl)(amino)carbene (CAAC) iminium gave a highly stable organic radical. The neutral paramagnetic species was fully characterised; the unpaired electron is delocalised on both the CAAC and the pyridine heterocycles. The results were recently published in Chem. Eur. J. (click here) and selected as “Hot Paper” (see also here).

Nice collaborative study with the group of Hansgeorg Schnöckel: since Mg+ ions are isoelectronic to Na atoms, an easy single electron transfer (SET) can be expected for MgBr. Even at 190 K, the radical MgBr (obtained via its sophisticated condensation) in a metastable solution transfers its electron to a diazadiene entity. A paramagnetic Mg(II) compound [MgBr(L1)˙]2 (L1 = DippN=C(Me)C(Me)=NDipp) is formed consisting of a singly reduced ligand. As shown by EPR investigations, the dimeric complex dissociates in ethereal solvents to two monomeric subunits. In addition, the complex can subsequently be reduced with potassium to furnish again a Mg(I) compound, namely [K(thf)3]2[Mg2(L1)2], which was reported previously. The results were recently published in Chem. Commun. (click here) and featured on the inside front cover of issue 99.

In a collaborative study, the one-electron oxidation of a series of diaryldichalcogenides was studied in the groups of Konrad Seppelt and Jens Beckmann. The electronic and structural properties of the radical cations [(2,6-Mes2C6H3E)2]˙+ (E = S, Se, Te) were probed by Jeff Harmer and our group with the aid of EPR spectroscopy and density functional theory calculations. The paper was recently published in Chem Sci. (click here) and belongs to the Top 25 most downloaded Chemical Science articles October-December 2014 (click here).

The preparation and structures of Tpm-based heterobimetallic complexes are reported, also including the first complexes containing Zn-Cr, Cd-Cr, and Cd-Mo bonds. The nature of the metal-metal bonds was probed by quantum chemical calculations. The results were recently published in Chem. Eur. J. (click here).

In a recent collaborative paper with several groups of the SFB 3MET we reported on a detailed theoretical and spectroscopic study on the electronically excited states of a highly symmetric, trinuclear palladium complex both in the gas phase and in solution. The results were published in Phys. Chem. Chem. Phys. (click here). In a follow-up paper, different time-resolved IR spectroscopic methods covering the femtosecond up to the microsecond range as well as density functional computations have been performed to unravel the structure and character of this complex in the electronically excited state. The results were also published in Phys. Chem. Chem. Phys. (click here).

The preparation and structures of coinage metal complexes of tris(pyrazolyl)methanide-based redox-active metalloligands are reported, together with density functional theory (DFT) calculations and detailed cyclic voltammetry studies in solution in order to elucidate a conceivable electronic interplay between the metal atoms. The results were recently published in Organometallics (click here).

Recently, the 5th German edition of the renowned Inorganic Chemistry textbook “Huheey” appeared, again with contributions from our side.
Huheey, James E. / Keiter, Ellen A. / Keiter, Richard L.
Anorganische Chemie
Hrsg. v. Steudel, Ralf
Bearb. v. Breher, Frank / Finze, Maik / Johrendt, Dirk / Lunk, Hans-Joachim / Kaupp, Martin / Radius, Udo / Schatzschneider, Ulrich
DeGruyter, 2014.

By using a multifunctional silyl ligand based on the tris(3,5-dimethylpyrazolyl)silyl scaffold [Si(3,5-Me2pz)3]–, we succeeded in obtaining the homoleptic tetrakis(silyl) complexes of Pd0 and Pt0, which were characterized by various methods, including multinuclear NMR spectroscopic techniques, cyclic voltammetry in combination with DFT calculations, X-ray crystallography, and EPR spectroscopy. The complexes form very unusual metal-centered heterocubane structures in the solid state and in solution. The rather rigid heterocubane ligand shells were found to facilitate the stepwise, quasi-reversible oxidation to their d9 MI and d8 MII analogues (click here; see also cover of issue 52 and spotlight on ChemistryViews).






